Wireless connectivity is the heart of modern healthcare from ICU monitors to home-based diagnostics. But in a world full of Wi-Fi, Bluetooth, and other wireless devices, medical devices share the environment with many other users and conflict is inevitable. So how can you ensure your device keeps performing when it matters most? That’s the aim of evaluating wireless coexistence: a vital concept in connected health technology.
In this post, we answer six essential questions medical device developers are asking about coexistence evaluation
What Is Wireless Coexistence?
Wireless coexistence is the ability of a system to maintain its wireless function in environments where other wireless systems are operating, especially in shared frequency bands like 2.4 GHz or 5 GHz.
This is different from EMC (electromagnetic compatibility) immunity. Coexistence focuses on the interaction between systems using the same spectrum, such as: Bluetooth Low Energy (BLE), Wi-Fi, ZigBee, and other devices operating in industrial, scientific, and medical (ISM) bands.
Why Should I Care?
Because dropped signals can lead to delayed diagnoses, missed alerts, or compromised safety.
If your device is responsible for transmitting patient data or triggering alarms, wireless failures can directly impact patient safety. Coexistence testing helps ensure reliability in real-world conditions, like a hospital ward full of Wi-Fi routers and smartphones. These considerations are clearly discussed in FDA’s wireless guidance document.
What FDA-Recognized Documents Should I Reference?
You’ll want to use these two FDA-recognized standards as your guide:
- AAMI TIR69 – Risk Management for RF Wireless Coexistence
- ANSI C63.27 – Standard for Evaluation of Wireless Coexistence
These provide a framework for risk categorization, testing methodology, and performance evaluation.
Do I Have to Test for Wireless Coexistence?
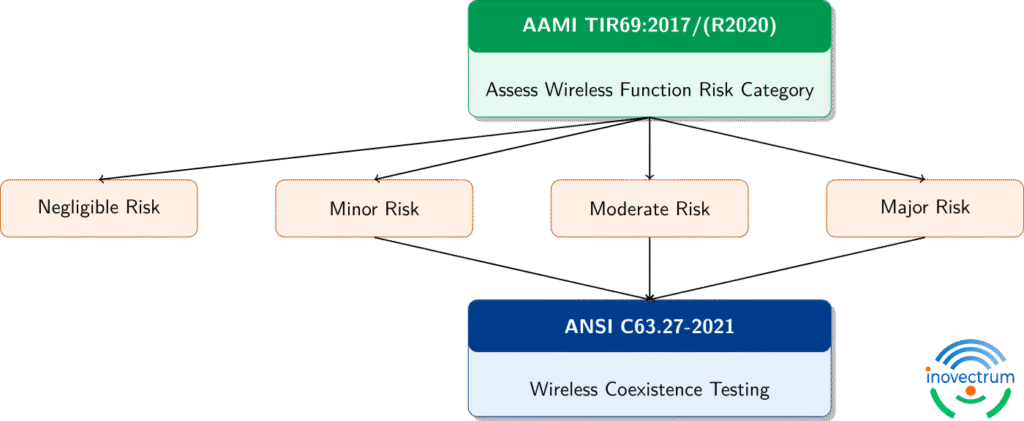
It depends on the risk of your wireless function. According to AAMI TIR69:
- Negligible risk: Testing is optional.
- Minor to Major risk: Testing is specified with a one-to-one mapping between the risk category and a testing tier in ANSI C63.27.
If failure of your wireless function could cause harm, delay therapy, or affect clinical decisions, testing becomes essential.
What’s Being Tested?
Coexistence testing examines your device’s Functional Wireless Performance (FWP), which can be specified through Key performance indicators (KPIs) like:
- Throughput
- Latency
- Connection stability
- Pairing success
FWP forms the basis of the test pass/fail criteria that will be used during testing to document test outcomes.
How Do I Use the Test Outcome?
Coexistence testing outcomes can be used to:
- Improve device hardware or firmware and investigate real-life use failures
- Define pass/fail thresholds for use scenarios
- Support risk mitigation claims in FDA submissions
- Provide user guidance (e.g., “Keep at least X meter(s) away from Wi-Fi routers”)
The test outcomes could also be used for more elaborate objectives like estimating the likelihood of wireless coexistence or extrapolating separation distance recommendations for deployment in homes, clinics, or hospitals.
Reliable wireless performance is essential in today’s connected healthcare environments, where interference is a constant reality. Evaluating wireless coexistence thoroughly gives developers the insight needed to design robust, patient-ready devices. It’s a practical step that strengthens product performance, supports regulatory success, and ultimately helps ensure technology works exactly when it’s needed most.
At Inovectrum, we help medtech teams design, evaluate, and launch wireless-enabled systems that thrive in complex environments. Reach out to learn how we can support your next connected health innovation.
