At Inovectrum, our perspective is shaped by years of leadership at the forefront of wireless healthcare innovation.
The seminars and publications featured here include work developed, delivered, or contributed to by our founder, Dr. Omar Al-Kalaa, in previous roles within federal research and regulatory environments. These contributions reflect the caliber of technical insight, regulatory engagement, and system-level thinking that Inovectrum brings to every client engagement today.
Listen to the Full Conversation: Wireless Coexistence and Medical Device Connectivity w. Dr. Omar Al-Kalaa
Featured Appearances
FDA Regulatory Science Tool—TRUST: A Testbed Design Model for Evaluating 5G-Enabled Medical Devices
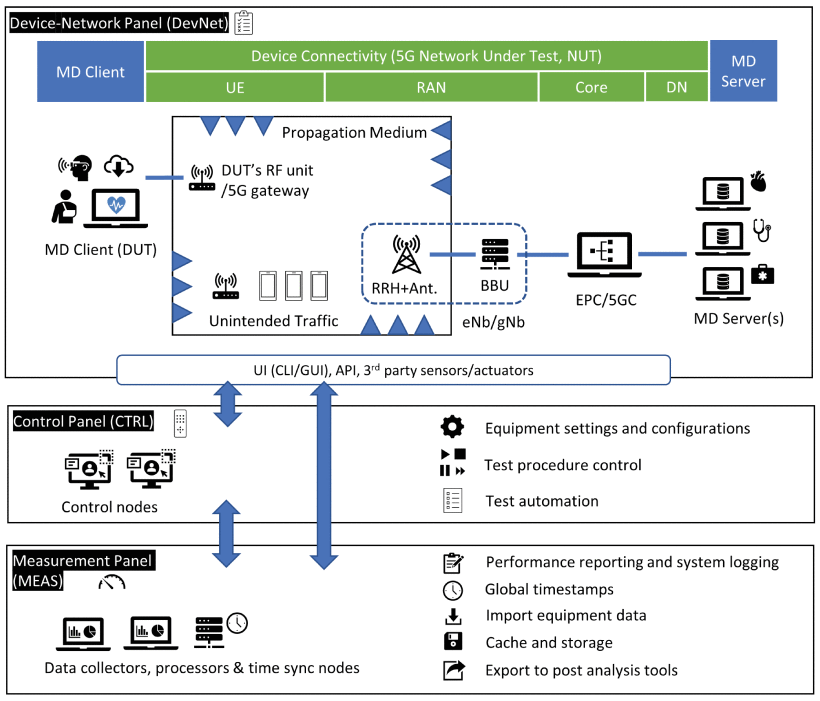
Source: Figure 2, “Testbed as a RegUlatory Science Tool (TRUST): A Testbed Design for Evaluating 5G-Enabled Medical Devices,” by Yongkang Liu and Mohamad Omar Al Kalaa, IEEE Access, vol. 11, pp. 81563-81576, 2023.
Testbed as a Regulatory Science Tool (TRUST) is a design model for creating 5G network testbeds to evaluate the data transmission of medical device functions over 5G connectivity. This tool enables users like medical device manufacturers and test labs to define their specific testing needs, which TRUST then translates into relevant testbed characteristics, procedures, and options. These options facilitate the planning and configuration of 5G network scenarios, the coordination and automation of tests across different testbed components, and the monitoring of medical device and 5G network performance to verify end-to-end connectivity. TRUST also identifies essential 5G components for application-relevant testing and guides users in selecting and customizing testbed elements. Its modular design offers controlled hardware, software, and environmental configurations, allowing users to replicate 5G setups and verify results independently, aided by comparison tables for determining necessary 5G features and evaluating implementation choices based on accuracy, efficiency, and cost.
Medical Device Innovation Consortium: Landscape Analysis of 5G in Healthcare

“The Landscape Analysis of 5G in Healthcare surveys the role of 5G connectivity in current and future applications within the healthcare continuum of care. The report provides an overview of 5G technology along with several healthcare use cases, such as 5G-enabled simulation with extended reality, 5G-enabled robotics, mobile units, and remote care. Key challenges and knowledge gaps identified must addressed to deliver the benefits of 5G in healthcare more safely to patients.”

Connect With Us
Inovectrum partners with medical device developers, telecommunications providers, testing laboratories, and policy leaders to advance the future of connected healthcare. Reach out by email, phone, or through the contact form — we look forward to connecting with you!

